Our Mission
Evaluate promising therapeutic approaches in multi-institutional clinical trials to improve the outcomes of blood and bone marrow transplantation and other cellular therapies for patients facing life-threatening blood disorders.
BMT CTN by the Numbers
- 125+
- Sites
- 60+
- Clinical Studies
Launched
- 16,800+
- Patients
Enrolled
- 605,000+
- Biospecimen
Aliquots
Created
- 170+
- Papers
Published
Updates made on April 1, 2024
BMT CTN at a Glance
- Investigators or Research Staff...
- Conducts paradigm changing research addressing important issues in transplantation and cellular therapy
- >50 studies opened at >150 transplant centers in 39 US states since inception in 2001
- Opportunities for investigators at all levels
- Patients or Caregivers...
- Improves care and outcomes for patients needing transplantation and other cellular therapies
- Conducts studies to evolve treatment options for patients with a wide variety of diseases
- Provides access to clinical research studies for patients at centers across the US
- Partners...
- Led by global experts in transplantation and cellular therapy who work together to achieve common goals
- Proven track record in protocol development, accrual and results dissemination
- Network includes the largest US transplant and cell therapy centers
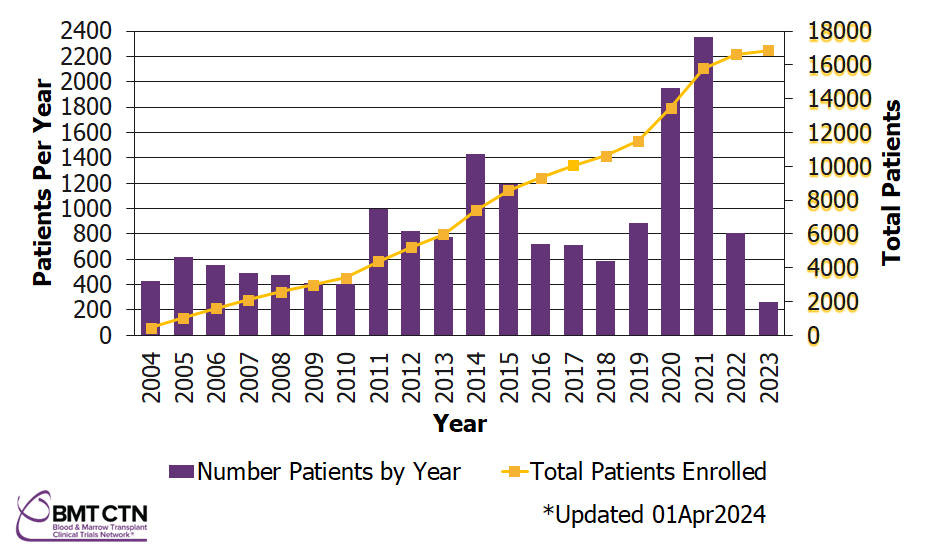
Number of Patients Enrolled on BMT CTN Studies
The BMT CTN is funded by the National Heart, Lung, and Blood Institute (NHLBI) and the National Cancer Institute (NCI), both parts of NIH (National Institutes of Health) with grants to:
Data and Coordinating Center: (U24HL138660), and to the Core and Consortia Sites: UG1HL069246, UG1HL069249, UG1HL069254, UG1HL069274, UG1HL069278, UG1HL069286, UG1HL069290, UG1HL069291, UG1HL069301, UG1HL069310, UG1HL069315, UG1HL069330, UG1HL108945, UG1HL108987, UG1HL109137, UG1HL109322, UG1HL109526, UG1HL138641 and UG1HL138645
